End-to-End Case Handling with Regulatory Confidence
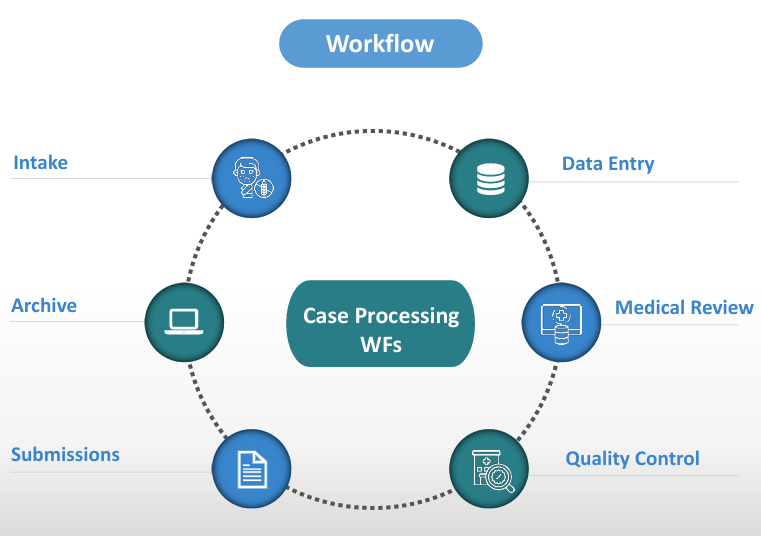
We deliver comprehensive post-marketing safety case management covering the complete ICSR and AEFI lifecycle—from intake and triage to medical review, quality control, regulatory submission, and follow-up. Our structured, compliant approach ensures data accuracy, timely reporting, and consistent safety oversight across global markets, supporting regulatory compliance and patient safety throughout the product lifecycle.
Case processing
Triage and case classification in line with regulatory requirements
Data entry and case processing by trained safety professionals
Quality control and medical review to ensure accuracy and compliance
Regulatory submission and reporting within defined timelines
Additional Safety Operations & Compliance Support
Pharmacovigilance Services
Contact Us
-
B04-332, Business Center 03, RAKEZ Business Zone-FZ,
Ras Al Khaimah, UAE - +971 55 895 5849
-
info@yashfin.net
info@yashfinpharma.com
Our Brochures
View our 2023 Medical prospectus of brochure for an easy to read guide on all of the services offer.
