Literature Monitoring & Surveillance Services
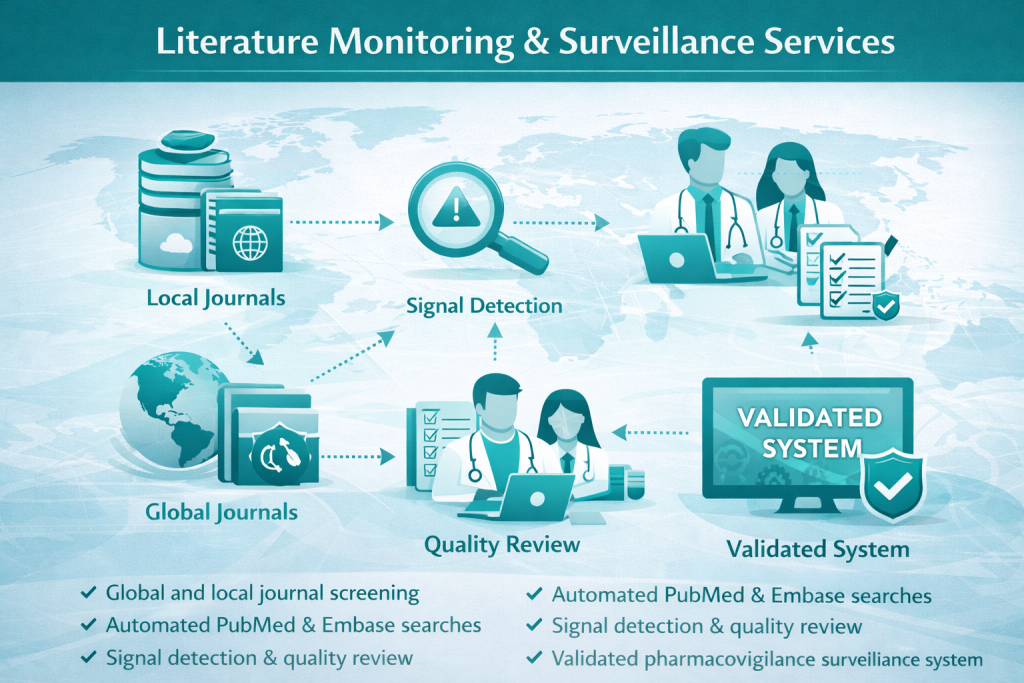
Yashfin offers comprehensive local and global literature monitoring and surveillance services to support early identification of safety signals and ensure continuous compliance with global pharmacovigilance regulations.
Our validated systems and quality-driven processes ensure accurate tracking, medical assessment, and full documentation of scientific literature.
Proven Scale:
500K+ scientific articles screened through validated literature surveillance workflows.
Scientific articles screened
0
K
Local Literature Monitoring
Surveillance of non-indexed local and regional biomedical journals, ensuring coverage beyond global databases.
Surveillance of non-indexed local and regional biomedical journals, ensuring coverage beyond global databases.
Validated Literature Databases
Use of validated local and central literature databases to ensure data integrity, traceability, and inspection readiness.
Use of validated local and central literature databases to ensure data integrity, traceability, and inspection readiness.
Automated Journal Coverage & Updates
Automated solutions for journal tracking, alerts, and update management, reducing manual dependency.
Automated solutions for journal tracking, alerts, and update management, reducing manual dependency.
Search Strategy Validation & Quality Review
Validated search strings with 100% quality review of identified hits to ensure completeness and accuracy.
Validated search strings with 100% quality review of identified hits to ensure completeness and accuracy.
Full-Text Access & Translation Support
Procurement of full-text articles with translation support for non-English publications.
Procurement of full-text articles with translation support for non-English publications.
Retrospective Literature Data Management
Backlog and retrospective data screening and documentation for regulatory compliance and remediation activities.
Backlog and retrospective data screening and documentation for regulatory compliance and remediation activities.
Qualified PV Literature Review Team
Experienced team responsible for screening, quality control (QC), medical assessment, and documentation.
Experienced team responsible for screening, quality control (QC), medical assessment, and documentation.
Integrated PV-QMS Oversight
In-built PV Quality Management System for deviation management, gap tracking, and continuous improvement.
In-built PV Quality Management System for deviation management, gap tracking, and continuous improvement.
Business Continuity & Backup Surveillance
Manual surveillance mechanisms and backup systems to ensure uninterrupted monitoring during system outages.
Manual surveillance mechanisms and backup systems to ensure uninterrupted monitoring during system outages.
A robust, validated, and inspection-ready literature surveillance framework that supports signal detection, regulatory compliance, and global pharmacovigilance obligations.
Pharmacovigilance Services
Contact Us
-
B04-332, Business Center 03, RAKEZ Business Zone-FZ,
Ras Al Khaimah, UAE - +971 55 895 5849
-
info@yashfin.net
info@yashfinpharma.com
Our Brochures
Explore our Service Prospectus for a clear, easy-to-read overview of all services we offer.
