Our Regulatory Intelligence Services
Yashfin’s Regulatory Intelligence for PV Services enables organizations to stay ahead of evolving global and local pharmacovigilance requirements through proactive surveillance, expert analysis, and structured dissemination of regulatory updates.
Our service supports informed decision-making, compliance planning, and timely implementation of regulatory changes across markets.
Proven Expertise:
40+ Regulatory Strategy engagements supporting global PV compliance.
Regulatory Strategy engagements
0
+
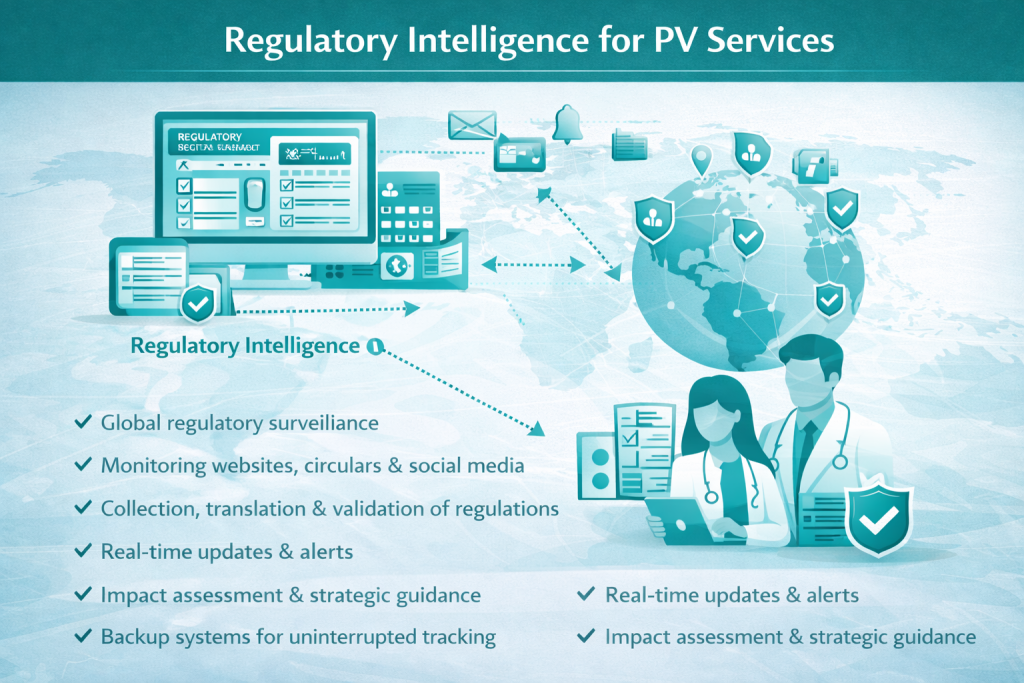
Regulatory Surveillance via Intelligence Platforms
Continuous monitoring using specialized regulatory intelligence software platforms to track global PV regulatory changes.
Continuous monitoring using specialized regulatory intelligence software platforms to track global PV regulatory changes.
Regulation Collection, Translation & Validation
Collection, translation, and validation of pharmacovigilance laws, guidelines, and safety-related regulatory updates.
Collection, translation, and validation of pharmacovigilance laws, guidelines, and safety-related regulatory updates.
Real-Time Regulatory Updates
24/7 access to real-time regulatory intelligence with country-specific reports and alerts.
24/7 access to real-time regulatory intelligence with country-specific reports and alerts.
Impact Assessment & Strategic Guidance
Review by qualified regulatory and PV experts to assess operational impact and provide practical compliance and strategy recommendations.
Review by qualified regulatory and PV experts to assess operational impact and provide practical compliance and strategy recommendations.
A proactive, inspection-ready regulatory intelligence framework that supports compliance, reduces regulatory risk, and strengthens global pharmacovigilance governance.
Pharmacovigilance Services
Contact Us
-
B04-332, Business Center 03, RAKEZ Business Zone-FZ,
Ras Al Khaimah, UAE - +971 55 895 5849
-
info@yashfin.net
info@yashfinpharma.com
Our Brochures
Explore our Service Prospectus for a clear, easy-to-read overview of all services we offer.
